Endoscopes Recalled Following Superbug Threat
Mid-January 2016 saw the conclusion of a Senate investigation into deadly infections being spread through improperly cleaned duodenoscopes. The Senate investigation found that a lack of governmental oversight combined with failures both during the manufacturing process and cleaning processes subjected patients to excessive and unnecessary risk of infections. The devices, which are used to peer into the small intestine to look for abnormalities, were used in thousands of procedures between 2012 and 2015. Some of the cases resulted in patients contracting infections and ultimately dying from them.(Article continues below Infographic)
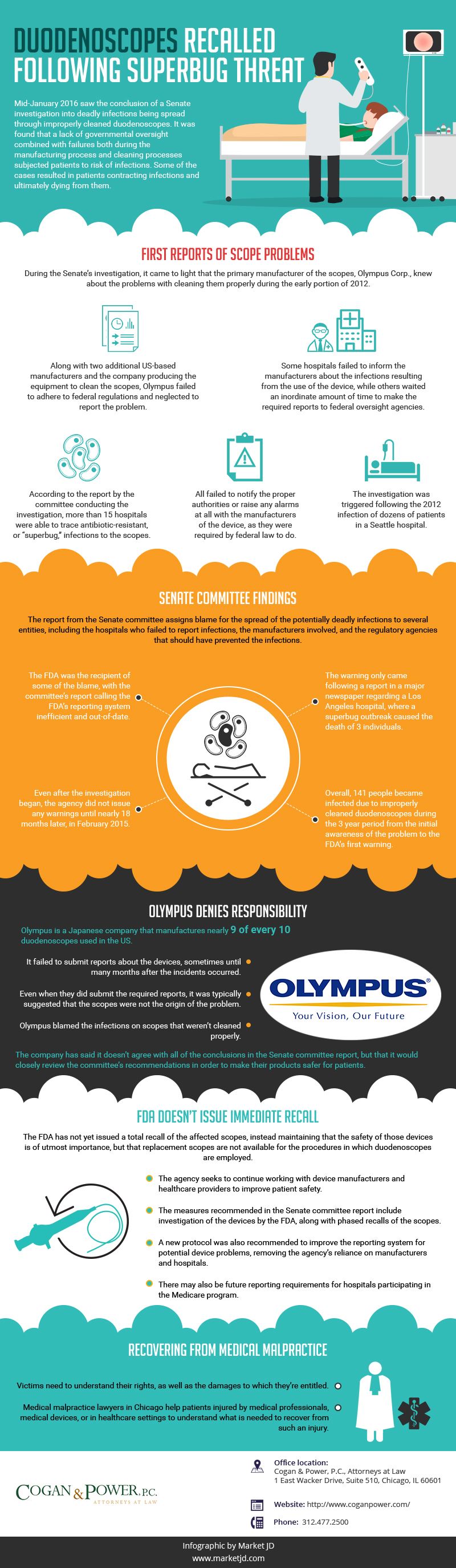
First Reports of Scope Problems
During the Senate’s investigation, it came to light that the primary manufacturer of the scopes, Olympus Corp., knew about the problems with cleaning them properly during the early portion of 2012. Along with two additional US-based manufacturers, as well as the company producing the equipment to clean the scopes, Olympus failed to adhere to federal regulations and neglected to report the problem. Some hospitals also failed to inform the manufacturers about the infections resulting from the use of the device, while others waited an inordinate amount of time to make the required reports to federal oversight agencies. These failures resulted in additional time that the scopes were in use and endangering patients.
According to the report by the committee conducting the investigation, more than fifteen hospitals were able to trace antibiotic-resistant, or “superbug,” infections to the scopes. All failed to notify the proper authorities or raise any alarms at all with the manufacturers of the device, as they were required by federal law to do. The investigation was triggered following the 2012 infection of dozens of patients in a Seattle hospital.
Senate Committee Findings
The report from the Senate committee assigns blame for the spread of the potentially deadly infections to several entities, including the hospitals who failed to report infections, the manufacturers involved, and the regulatory agencies that should have prevented the infections. The Food and Drug Administration (FDA) was the recipient of some of the blame, with the committee’s report calling the FDA’s reporting system inefficient and out-of-date. The FDA failed to investigate any reports until September of 2013. Even after the investigation began, the agency did not issue any warnings until nearly eighteen months later, in February 2015. The warning only came following a report in a major newspaper regarding a Los Angeles hospital, where a superbug outbreak was credited with the deaths of three individuals. Overall, 141 people became infected due to improperly cleaned duodenoscopes during the three year period from the initial awareness of the problem to the FDA’s first warning.
Olympus Denies Responsibility
FDA rules require manufacturers of medical devices to report any adverse health impacts that are related to the products they manufacture. Olympus, a Japanese company that manufactures nearly nine of every ten duodenoscopes used in the US, failed to submit reports about the devices, sometimes until many months after the incidents occurred. Even when they did submit the required reports, it was typically suggested that the scopes were not the origin of the problem. Rather, Olympus blamed the infections on scopes that weren’t cleaned properly. The company has said it doesn’t agree with all of the conclusions in the Senate committee report, but that it would closely review the committee’s recommendations in order to make their products safer for patients.
FDA Doesn’t Issue Immediate Recall
The FDA has not yet issued a total recall of the affected scopes, instead maintaining that the safety of those devices is of utmost importance, but that replacement scopes are not available for the procedures in which duodenoscopes are employed. The agency instead seeks to continue working with device manufacturers and healthcare providers to improve patient safety. The measures recommended in the Senate committee report include investigation of the devices by the FDA, along with phased recalls of the scopes. A new protocol was also recommended to improve the reporting system for potential device problems, removing the agency’s reliance on manufacturers and hospitals. There may also be future reporting requirements for hospitals participating in the Medicare program.
Recovering from Medical Malpractice
Doctors, pharmacists, nurses, and other medical professionals are entrusted with the lives of their patients. There is an expectation that those patients will receive, at minimum, a basic standard of care. As this situation with medical scopes illustrates, there are many failures that can result in injury to patients. Medical malpractice lawyers in Chicago help protect the rights of those who have suffered medical malpractice. Victims need to understand their rights, as well as the damages to which they’re entitled. The damage caused by medical malpractice can be emotionally, mentally, physically, and financially devastating. Medical malpractice lawyers in Chicago help patients injured by medical professionals, medical devices, or in healthcare settings to understand what is needed to recover from such an injury.
